A 70-year-old system could help us prepare a bird flu vaccine for humans
The widespread presence of bird flu in US cattle and milk has vaccine scientists on high alert.
Avian influenza is no newcomer in human history. In 1918, a strain ripped through war-stricken populations, resulting in the deaths of more than 50 million people – more than any other recorded disease outbreak. Today, a highly pathogenic strain of bird flu, named H5N1, is causing a global animal pandemic, though human infection with this strain has so far been very rare.
Since the strain's emergence in 1996, it has led to the deaths of billions of poultry birds, killed millions of birds in the wild and infected at least 48 species of mammals. In the US, farm cats have died after drinking the unpasteurised milk of H5N1-infected cattle. (Read more about how bird flu became an animal pandemic.)
The latest strain has not adapted to spread easily between humans. Since 2003, 463 people have died from the virus, which has a fearsome mortality rate of over 50% among humans. So far, cases of human-to-human transmission are thought to have been limited and non-sustained.
But the virus's ability to jump from mammal to mammal may be changing.
In March, the variant was found to be spreading among cattle as well as poultry. By April, a farm worker in Texas became the first person to contract the virus from a cow. With modern cattle farming offering a potentially vast incubator for the virus, further mutation that results in more efficient spread in mammals, including humans, could become more likely, scientists have warned.
If the virus does gain the ability to spread efficiently in humans, a vaccine would be one way to slow its progress. According to the World Health Organization (WHO), the virus is not yet at a stage that requires a human vaccine. But in anticipation of such a change, the organisation has confirmed that there are systems and plans in place to produce one.
BBC Future Planet spoke to Maria Van Kerkhove, an epidemiologist and interim director of epidemic and pandemic preparedness and prevention at WHO, about what the process of producing and rolling out a human vaccine for bird flu might look like.
Has avian influenza's circulation among US cattle – as well as its detection in milk – made the need for a human vaccine more pressing?
Anytime that new species are infected, it changes our understanding of the virus: the more these viruses circulate, the more opportunities they have to change, and to infect people.
Right now, we're seeing herd-to-herd transmission within cattle in the US – and there are a lot of studies underway that are trying to understand how the virus is circulating between the farms. On the H5N1 virus's presence in milk, we're also working with a number of researchers who are looking at the role of pasteurisation and virus inactivation. So for now it's really important for people to consume only pasteurised milk products.
But human vaccines are for humans. And (at point of interview) we've had only 28 reported human cases since 2021, and no documented human-to-human transmission. For WHO to trigger increased development or the production of H5N1 vaccines, then something would really need to change in terms of the risk in humans.
At what point would the rollout of a human vaccine for bird flu become necessary?
To evaluate transmissibility and severity, and especially if the virus were to more efficiently transmit between people, the WHO regularly undertakes risk assessments of all available data. As well as the virus's internal, genetic make-up, the assessment would consider the phenotypic characteristics of the virus, i.e. how it operates in the world. Would it be transmitted only among people who are in close contact with one another, such as health workers? Would it lead to small clusters of infections? Or efficient transmission with large outbreaks?
What systems are in place to deal with a rollout?
Unlike with Covid-19, which stemmed from the Sars-CoV-2 virus, we have a 70-year system in place for studying influenza viruses.
Called the Global Influenza Surveillance and Response System (GISRS), this global partnership entails 150 national influenza centres, in 130 member states – including 12 centres that are specific to H5. These centres are already looking at the circulation of influenza viruses to see which subtypes could potentially become epidemics, and which could potentially become pandemics.
We then have another system, called the Pandemic Influenza Preparedness framework (PIP), that, together with GISRS, identifies candidate vaccine viruses so the potential production of vaccines can start ahead of time. In that framework, we already have several H5N1 vaccines that are in development.
Scaling up vaccine development and production would require a declaration by WHO – based on the above assessments – that we have a virus that has pandemic potential. When that happens, we have agreements that are in place to switch production from seasonal influenza vaccine production to pandemic vaccine production.
But we're not there yet. We're not seeing human-to-human transmission.
Are vaccine makers prepared for an avian influenza mutation that could transmit efficiently between humans?
When you have a novel pathogen, then the whole world is susceptible to infection. That's what we saw with Sars-CoV-2.
We don't yet know what the susceptibility of the population to H5N1 will be. There have been surveys done on H5N1 to see how many people have actually been exposed to this virus – and so far the only people to fall ill are those who have been directly exposed through their occupations, such as contact with poultry.
With this particular virus, I think the world could be largely susceptible to it. But what I want to reiterate is that we have a global system in place that is tracking these viruses.
We also have a number of viruses that we call "candidate vaccine viruses", which are available to manufacturers to allow them to develop vaccines. Among these, we have two available candidate vaccine viruses that are related to the H5N1 virus that is circulating right now. So we're in a very good position to ramp up production should we need to.
Would existing vaccines work if the virus further adapts and mutates?
If the virus changes, we have a system in place to recommend that new virus for manufacturers to use.
We also work with partner agencies at the World Organisation for Animal Health and the Food and Agricultural Organization of the UN, and their veterinary labs, that are sharing viruses from animal species. And we're constantly looking at these influenza viruses to ask: are they a different subtype? Are they more prevalent? Where are they being detected? And could this avian influenza virus become more transmissible and become a pandemic?
How long would it take to roll out a bird flu vaccine for humans?
Current vaccines for avian influenza do not use the same messenger ribonucleic acid (mRNA) technology that was used for Covid-19. Those vaccines help the body to produce its own vaccine antigens (inactivated virus proteins), and can be manufactured at speed.
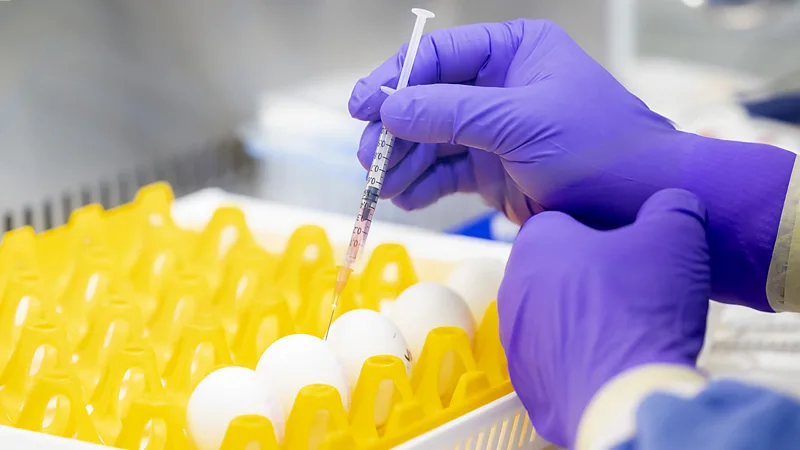
In contrast, current influenza vaccines use a slower, more traditional egg-based manufacturing process. Egg-based vaccines involve injecting the candidate vaccine viruses into fertilised eggs, incubating them so the virus replicates in weaker form, and then harvesting the resulting fluid.
We estimate that four to eight billion doses of a pandemic influenza vaccine could be produced over a 12-month period. Through our PIP framework, and the agreements we have with vaccine manufacturers, the WHO would have access to about 11-12% of production right away, which we could use based on need. That's approximately 500 million doses that we would have access to (though that amount would vary depending on antigen needs).
How does that compare with how the Covid-19 vaccines rolled out?
Covid-19 vaccines took about a year to be used. A potential vaccine for avian influenza could be available earlier than that because we're more prepared. This is because we have identified and made available the H5N1 candidate vaccine viruses, and we have an existing influenza vaccine manufacturing pipeline with these agreements in place.
In terms of use of vaccines, we would look at who is most likely to be infected in terms of exposure, and those who are most likely to experience severe disease – taking into consideration risk factors like age, pregnancy or underlying conditions. Young children are particularly at risk of influenza viruses.
What can governments do to help scientists prepare a potential human vaccination programme?
We're asking governments to increase their surveillance in animals and humans, especially those who are exposed to infected animals. And to ensure that they're sequencing the virus and sharing those sequences, so we can really understand any changes.
They also need to reinforce and sustain surveillance and vaccine-delivery systems that were strengthened during Covid-19, rather than directing money away from infectious diseases to other threats. Plus, there's a lot governments can do to increase trust in vaccines, and especially to address misinformation and disinformation.
How can misinformation about vaccines best be addressed?
This requires a multi-faceted approach and needs to look at the source of the misinformation or disinformation. During Covid-19, there was work done by some of the big online platforms to prevent this information spreading – but some of that has now stopped. So it's really up to organisations like ours and national governments to try to make sure that what is online is accurate.
-bbc






